Lewis Electron Dot Structure Calculator
- Lewis Electron Dot Structure Calculator Form
- Lewis Electron Dot Structure Calculator Answer
- Lewis Electron Dot Structure Calculator Formula
- Lewis Electron Dot Structure Calculator Solver
- Lewis Electron Dot Structure Calculator Equation
- Lewis Electron Dot Structure Calculator
Learning Objectives
Calculate the formal charge of the ligand atoms to complete the Lewis structure. If the structure is not correct, calculate the formal charge on each of the ligand atoms. Then to obtain the correct structure, form a multiple bond by sharing an electron pair from the. Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the. In 1916, ten years before the Schrodinger wave equation, G. Lewis suggested that a chemical bond involved sharing of electrons. He described what he called the cubical atom, because a cube has 8 corners, to represent the outer valence shell electrons which can be shared to create a bond.This was his octet rule. Rules for drawing Lewis dot structures.
By the end of this section, you will be able to:
- Write Lewis symbols for neutral atoms and ions
Lewis Symbols of Monoatomic Elements
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.)
For example, the Lewis electron dot diagram for calcium is simply
Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.
Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur:Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds.Figure 2. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change.
Example 1: Writing Lewis DoT SYmbols of Elements
What is the Lewis electron dot diagram for each element?
- aluminum
- selenium

The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons (or three single dots around the atom):
The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows:
Check Your Learning
What is the Lewis electron dot diagram for each element?
- phosphorus
- argon
Example 2: Writing Lewis DoT SYmbols of Ions
What is the Lewis electron dot diagram for each ion?
- Ca2+
- O2−
Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca2+.
Ca2+
The O2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows:
Check Your Learning
The valence electron configuration of thallium, whose symbol is Tl, is 6s25d106p1. What is the Lewis electron dot diagram for the Tl+ ion?
Key Takeaways
- Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
- Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.
Exercises
1. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
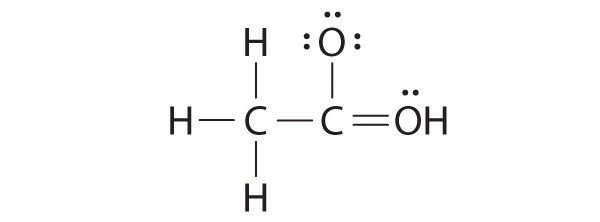
2. Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol?
3. What column of the periodic table has Lewis electron dot diagrams with two electrons?
4. What column of the periodic table has Lewis electron dot diagrams that have six electrons in them?
5. Draw the Lewis electron dot diagram for each element.
Lewis Electron Dot Structure Calculator Form
a) strontium
b) silicon
6. Draw the Lewis electron dot diagram for each element.
a) krypton
b) sulfur

7. Draw the Lewis electron dot diagram for each element.
a) titanium
b) phosphorus
8. Draw the Lewis electron dot diagram for each element.
a) bromine
b) gallium
9. Draw the Lewis electron dot diagram for each ion.
a) Mg2+
b) S2−
10. Draw the Lewis electron dot diagram for each ion.
a) In+
b) Br−
11. Draw the Lewis electron dot diagram for each ion.
a) Fe2+
b) N3−
12. Draw the Lewis electron dot diagram for each ion.
a) H+
b) H−
 Show Select Answer
Show Select Answer1. The first two electrons in a valence shell are s electrons, which are paired.
3. The second column of the periodic table
5.
a)
b)
7.
a)
b)
9.
a) Mg2+
b)
11.
Lewis Electron Dot Structure Calculator Answer
a) Fe2+
b)
Learning Objectives
- State the octet rule.
- Define ionic bond.
- Draw Lewis structures for ionic compounds.
In Section 4.7 we saw how ions are formed by losing electrons to make cations or by gaining electrons to form anions. The astute reader may have noticed something: many of the ions that form have eight electrons in their valence shell. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons in their original valence shell; the lower shell, now the valence shell, has eight electrons in it, so the atom becomes positively charged. For whatever reason, having eight electrons in a valence shell is a particularly energetically stable arrangement of electrons. The octet rule explains the favorable trend of atoms having eight electrons in their valence shell. When atoms form compounds, the octet rule is not always satisfied for all atoms at all times, but it is a very good rule of thumb for understanding the kinds of bonding arrangements that atoms can make.
It is not impossible to violate the octet rule. Consider sodium: in its elemental form, it has one valence electron and is stable. It is rather reactive, however, and does not require a lot of energy to remove that electron to make the Na+ ion. We could remove another electron by adding even more energy to the ion, to make the Na2+ ion. However, that requires much more energy than is normally available in chemical reactions, so sodium stops at a 1+ charge after losing a single electron. It turns out that the Na+ ion has a complete octet in its new valence shell, the n = 2 shell, which satisfies the octet rule. The octet rule is a result of trends in energies and is useful in explaining why atoms form the ions that they do.
Now consider an Na atom in the presence of a Cl atom. The two atoms have these Lewis electron dot diagrams and electron configurations:
[mathbf{Na, cdot }; ; ; ; ; ; ; ; ; ; mathbf{cdot }mathbf{ddot{underset{.: .}Cl}}mathbf{: :}]
[left [ Ne right ]3s^{1}; ; ; ; left [ Ne right ]3s^{2}3p^{5}]
For the Na atom to obtain an octet, it must lose an electron; for the Cl atom to gain an octet, it must gain an electron. An electron transfers from the Na atom to the Cl atom:
[mathbf{Na, cdot }curvearrowright mathbf{cdot }mathbf{ddot{underset{.: .}Cl}}mathbf{: :}]
resulting in two ions—the Na+ ion and the Cl− ion:
[mathbf{Na}^{+}; ; ; ; ; ; ; ; mathbf{:}mathbf{ddot{underset{.: .}Cl}}mathbf{: :}^{-}]
[left [ Ne right ]; ; ; ; ; left [ Ne right ]3s^{2}3p^{6}]
Lewis Electron Dot Structure Calculator Formula
Both species now have complete octets, and the electron shells are energetically stable. From basic physics, we know that opposite charges attract. This is what happens to the Na+ and Cl− ions:
[mathbf{Na}^{+}; + ; mathbf{:}mathbf{ddot{underset{.: .}Cl}}mathbf{: :}^{-}rightarrow Na^{+}Cl^{-}; ; or; ; NaCl]
where we have written the final formula (the formula for sodium chloride) as per the convention for ionic compounds, without listing the charges explicitly. The attraction between oppositely charged ions is called an ionic bond, and it is one of the main types of chemical bonds in chemistry. Ionic bonds are caused by electrons transferring from one atom to another.
In electron transfer, the number of electrons lost must equal the number of electrons gained. We saw this in the formation of NaCl. A similar process occurs between Mg atoms and O atoms, except in this case two electrons are transferred:
The two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond:
[mathbf{Mg,}^{2+}; + ; left[mathbf{:}mathbf{ddot{underset{.: .}O}}mathbf{: :}right]^{2-}; ; ; ; ; Mg^{2+}O^{2-}; or; MgO]
Remember, in the final formula for the ionic compound, we do not write the charges on the ions.
What about when an Na atom interacts with an O atom? The O atom needs two electrons to complete its valence octet, but the Na atom supplies only one electron:
[mathbf{Na, cdot }curvearrowright mathbf{cdot }mathbf{ddot{underset{.}O}}mathbf{: :}]
The O atom still does not have an octet of electrons. What we need is a second Na atom to donate a second electron to the O atom:
These three ions attract each other to give an overall neutral-charged ionic compound, which we write as Na2O. The need for the number of electrons lost being equal to the number of electrons gained explains why ionic compounds have the ratio of cations to anions that they do. This is required by the law of conservation of matter as well.
Example (PageIndex{1}): Synthesis of calcium chloride from Elements
With arrows, illustrate the transfer of electrons to form calcium chloride from (Ca) atoms and (Cl) atoms.
Solution
A (Ca) atom has two valence electrons, while a (Cl) atom has seven electrons. A (Cl) atom needs only one more to complete its octet, while (Ca) atoms have two electrons to lose. Thus we need two (Cl) atoms to accept the two electrons from one (Ca) atom. The transfer process looks as follows:
The oppositely charged ions attract each other to make CaCl2.
Exercise (PageIndex{1})
With arrows, illustrate the transfer of electrons to form potassium sulfide from (K) atoms and (S) atoms.
- Answer
Lewis Electron Dot Structure Calculator Solver
Summary
- The tendency to form species that have eight electrons in the valence shell is called the octet rule.
- The attraction of oppositely charged ions caused by electron transfer is called an ionic bond.
- The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions.
Lewis Electron Dot Structure Calculator Equation
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Lewis Electron Dot Structure Calculator
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)